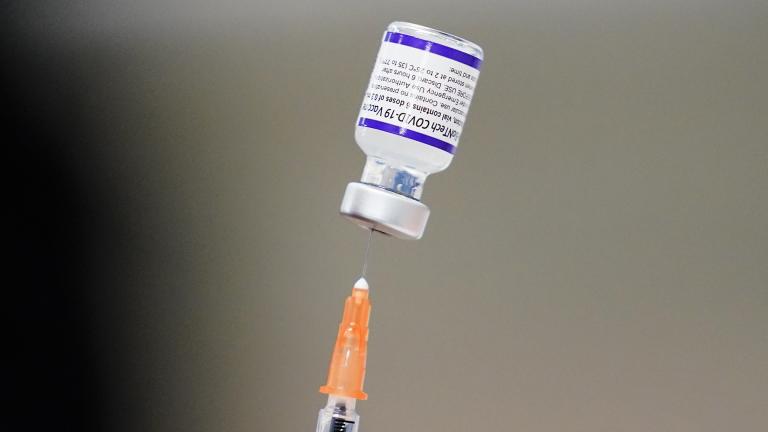
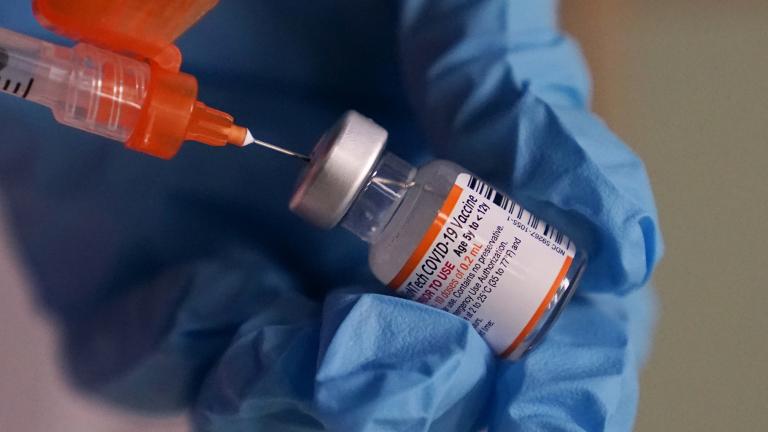
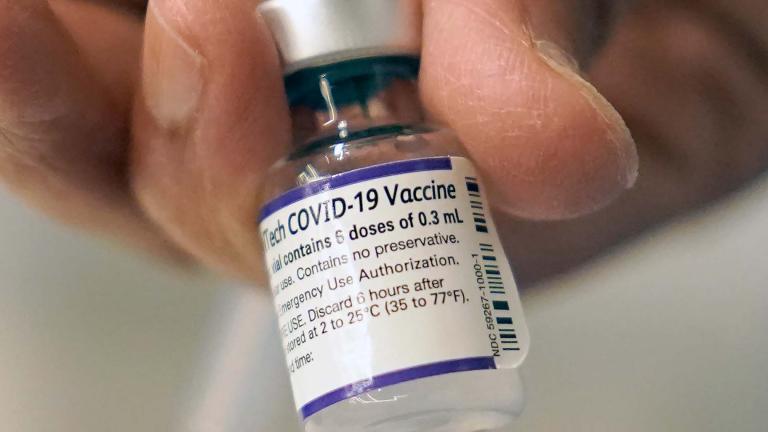
The move would add a fourth dose to the COVID vaccine regimen, which currently consists of a primary series of two shots, followed months later by a booster dose, in an effort to provide maximum protection to the over-65 population that has been hit hardest by the pandemic.
Pfizer
Friday, the FDA reversed course and said it had become clear the agency needed to wait for data on how well a third shot works for the youngest age group. Pfizer said in a statement that it expected the data by early April.
Pfizer is enrolling healthy adults to test a reformulated COVID-19 vaccine that matches the hugely contagious omicron variant, to see how it compares with the original shots.
The Centers for Disease Control and Prevention's advisers voted that a booster was safe for younger teens and should be offered to them once enough time — five months — has passed since their last shot.
The long-awaited milestone comes as U.S. cases, hospitalizations and deaths are all rising and health officials warn of a tsunami of new infections from the omicron variant that could overwhelm hospitals.
Pfizer said Monday its COVID-19 vaccine works for children ages 5 to 11 and that it will seek U.S. authorization for this age group soon — a key step toward beginning vaccinations for youngsters.
President Joe Biden says people who have been waiting for the FDA to formally approve a COVID-19 vaccine should get their shot now to stem what he calls a “pandemic of the unvaccinated.” Dr. Michael Angarone of Northwestern Medicine weighs in on that and more.
The U.S. gave full approval to Pfizer’s COVID-19 vaccine Monday, a milestone that could boost public confidence in the shots and spur more companies, universities and local governments to make vaccinations mandatory.
Just because Pfizer wants to offer COVID-19 vaccine boosters doesn’t mean people will be lining up anytime soon — U.S. and international health authorities say that for now, the fully vaccinated seem well protected.
Pfizer says it plans to meet with top U.S. health officials Monday to discuss the drugmaker’s request for federal authorization of a third dose of its COVID-19 vaccine as President Joe Biden’s chief medical adviser acknowledged that “it is entirely conceivable, maybe likely” that booster shots will be needed.
U.S. regulators on Monday expanded the use of Pfizer’s COVID-19 vaccine to children as young as 12, offering a way to protect the nation’s adolescents before they head back to school in the fall and paving the way for them to return to more normal activities.
Pfizer’s vaccine is authorized for ages 16 and older. Vaccinating children of all ages will be critical to stopping the pandemic — and helping schools, at least the upper grades, start to look a little more normal after months of disruption.
The biggest vaccination campaign in U.S. history kicked off Monday as health workers rolled up their sleeves for shots to protect them from COVID-19 and start beating back the pandemic — a day of optimism even as the nation’s death toll closed in on 300,000.
The first of many freezer-packed COVID-19 vaccine vials made their way to distribution sites across the United States on Sunday, as the nation’s pandemic deaths approached the horrifying new milestone of 300,000.
The nation’s first COVID-19 vaccine will begin arriving in states Monday morning, U.S. officials said Saturday, after the government gave the final go-ahead to the shots needed to end an outbreak that has killed nearly 300,000 Americans.
The U.S. gave the final go-ahead Friday to the nation’s first COVID-19 vaccine, marking what could be the beginning of the end of an outbreak that has killed nearly 300,000 Americans.