The Illinois Food Safety Act passed on a 37-15 bipartisan vote and will head to the state House for consideration. The banned chemicals are used in a wide variety of food products.
FDA
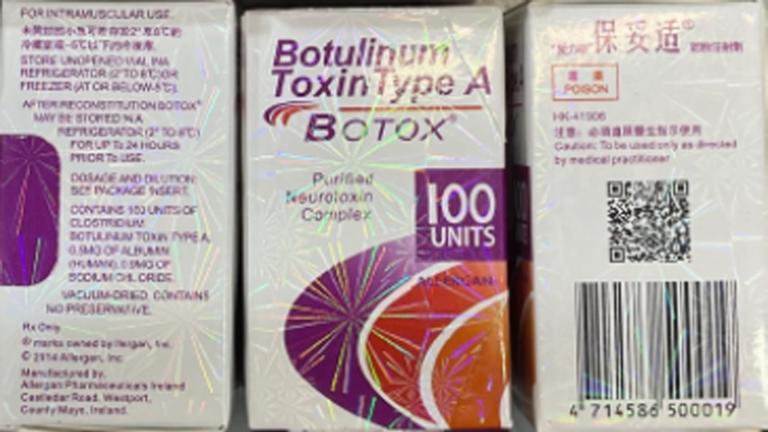
Some serious symptoms – including blurred vision, difficulty swallowing, shortness of breath, difficulty lifting one’s head and even hospitalizations – have been linked to the use of counterfeit Botox, the FDA said on Tuesday. As of Friday, a total of 19 women from nine states reported “harmful reactions.”
The lawsuit is the latest effort to force the government to ban menthols, which are disproportionately used by Black smokers and young people. It comes amid growing concerns from advocates that the federal plan could be derailed by election-year politics.
About 5,000 Illinoisans live with sickle cell disease, a gene defect most common in Black people that causes red blood cells to be misshapen and die off early, resulting in chronic fatigue and pain.
The drug’s approval came despite some concerns by FDA scientists about the company’s results, including whether women with certain medical conditions would understand that they shouldn’t take the drug.
An estimated 17 million people in the U.S. have the type of food allergies that can cause rapid, serious symptoms, including severe, whole-body reactions that are potentially deadly.
A group of Illinois lawmakers have a proposal that would ban a handful of common additives in food made and sold in Illinois starting in 2027. California last year became the first state to ban the substances that are common in mass-produced and ultra-processed cereals, candies, salad dressings and sodas.
Regulators on Friday approved two gene therapies for sickle cell disease that doctors hope can cure the painful, inherited blood disorder that afflicts mostly Black people in the U.S.
The FDA has been officially exploring the possibility of a menthol ban for more than a decade. In July 2013, the FDA requested comments on preliminary research, data and evaluations with regard to the regulation of menthol.
Scientists have long identified an association between the use of hair-straightening chemical products with an increased risk of certain cancers. Research suggests that about 50% of products advertised to Black women contain these types of chemicals, compared with about 7% that are advertised to White women.
The agency said the products are illegally marketed to treat conditions including conjunctivitis — known as pink eye — glaucoma and cataracts, and some of the warnings cited sterility issues with the products.
The leading decongestant used by millions of Americans is likely no better than a dummy pill, according to government experts who reviewed the latest research on the long-questioned drug ingredient.
A US Centers for Disease Control and Prevention advisory group is scheduled to meet to discuss COVID-19 vaccines Tuesday, meaning the vaccines could become available within just a few days,
The U.S. Food and Drug Administration is expected to give its nod to the updated vaccines in a few weeks. The announcement comes amid a late summer uptick in COVID-19.
Aspartame is a popular artificial sweetener found in thousands of products like diet sodas and sugar-free gum. It’s considered one of the most studied food additives in existence.
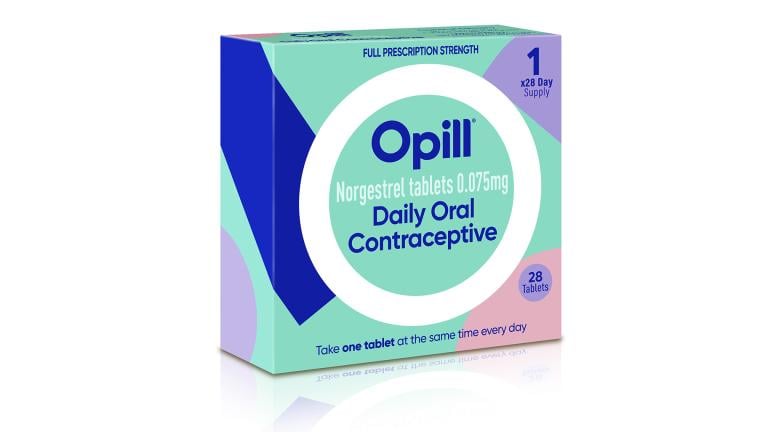
The Food and Drug Administration said Thursday it cleared Perrigo’s once-a-day Opill to be sold without a prescription, making it the first such medication to be moved out from behind the pharmacy counter.