Federal health advisers said a decades-old birth control pill should be sold without a prescription, paving the way for a likely U.S. approval of the first over-the-counter contraceptive medication. Currently, a prescription is required in the U.S.
FDA
In recent months, unexpected demand spikes, manufacturing problems and tight ingredient supplies have contributed to shortages that stress patients, parents and doctors.
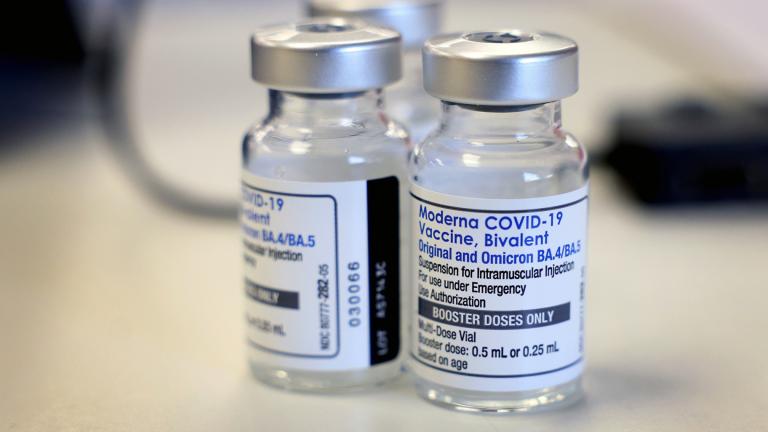
On Tuesday, the FDA changed the terms of the authorizations for those vaccines so that certain individuals could get an additional dose ahead of most others.
In an order signed by Justice Samuel Alito, the court put a five-day pause on the fast-moving case so the justices can decide whether lower court rulings restricting the FDA’s approval of the drug should be allowed to take effect in the short term.
Mifepristone was approved for use by the FDA more than two decades ago. In a ruling Friday, a federal judge in Texas blocked the FDA’s approval of the drug. At virtually the same time, a judge in Washington state ordered the FDA not to do anything that might affect the drug’s availability.
Revolution Farms of Caledonia, Michigan, voluntarily recalled lettuce produced and sold under the brand Revolution Farms on April 5, 2023, due to the potential for listeria contamination.
The U.S. Food and Drug Administration on Wednesday approved selling the leading version of naloxone without a prescription, setting the overdose-reversing drug on course to become the first opioid treatment drug to be sold over the counter.
The medication has been used by millions of Americans since the FDA granted it emergency use authorization in late 2021. The agency has the final say on giving Pfizer’s drug full approval and is expected to decide by May.
The Food and Drug Administration on Friday announced draft guidelines that would do away with the current three-month abstinence requirement for donations from men who have sex with men. Instead, all potential donors would be screened with a new questionnaire that evaluates their individual risks for HIV.
The Food and Drug Administration on Monday proposed a simplified approach for future vaccination efforts, allowing most adults and children to get a once-a-year shot to protect against the mutating virus.
The Food and Drug Administration’s decision aims to better protect the littlest kids from severe COVID-19 at a time when children’s hospitals already are packed with tots suffering from a variety of respiratory illnesses.
Philips initially estimated it could repair or replace the units within a year. But with the recall expanding to more than 5 million devices worldwide, the Dutch company now says the effort will stretch into 2023.
The Food and Drug Administration has given a green light for elementary school-age kids to get the updated booster doses — one made by Pfizer for 5- to 11-year-olds, and a version from rival Moderna for those as young as 6.
The move by the Food and Drug Administration tweaks the recipe of shots made by Pfizer and rival Moderna that already have saved millions of lives. The hope is that the modified boosters will blunt yet another winter surge.
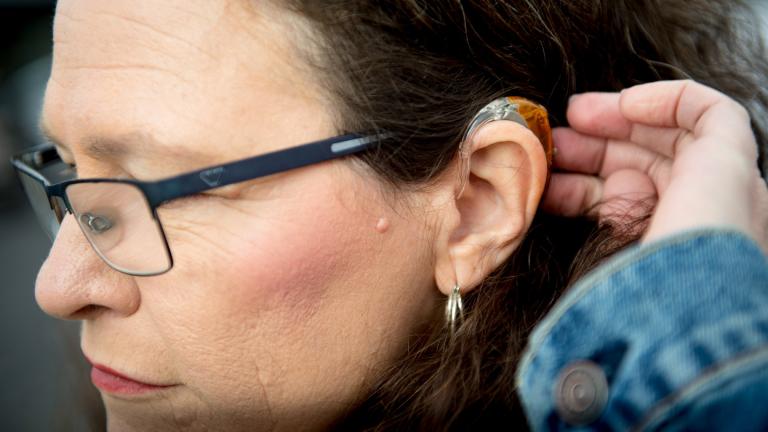
The regulation creates a new class of hearing aids that don’t require a medical exam, a prescription and other specialty evaluations, the Food and Drug Administration said. That’s expected to increase competition and eventually lower costs. The devices will be sold online or over-the-counter at pharmacies and other retail stores.
The FDA said Juul must stop selling its vaping device and its tobacco and menthol flavored cartridges. Those already on the market must be removed. Consumers aren’t restricted from having or using Juul’s products, the agency said.