Through desperate efforts to save their lives, scientists now better understand how to treat and prevent the disease — and millions of others may survive.
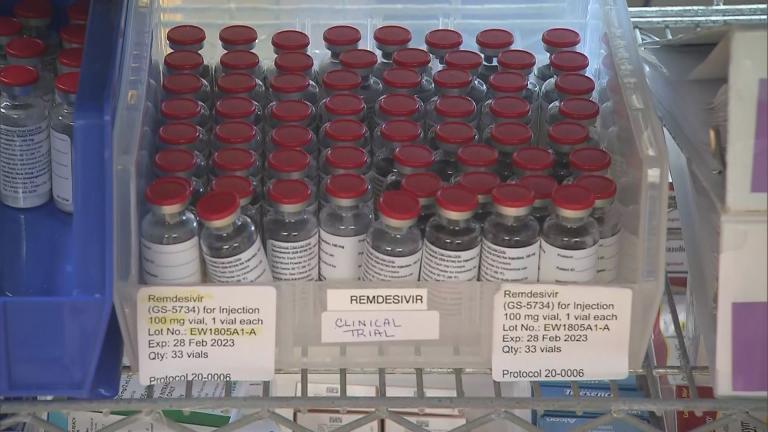
Remdesivir
Chicago’s Roseland Community Hospital has been on the front lines of the pandemic. But it wasn’t included in Illinois’ recent distribution of remdesivir, a move that’s angered hospital officials.
A select group of Illinois hospitals can now treat patients with the only drug so far authorized by the FDA as a treatment for COVID-19. Now the question is: Exactly who will get remdesivir?
The only drug given emergency authorization by the Food and Drug Administration to treat patients with the coronavirus has arrived in Illinois. But there’s not enough to go around – in Illinois or elsewhere.
The federal government is sending supplies of the first drug that appears to help speed the recovery of some COVID-19 patients to six states, where it will be distributed by health departments.
The FDA said in a statement that Gilead Science’s intravenous drug would be specifically indicated for hospitalized patients with “severe disease,” such as those experiencing breathing problems requiring supplemental oxygen or ventilators.
The nation’s top infectious disease expert said Thursday that new cases of the coronavirus are a certainty as states begin to roll back restrictions.
A biotech company says its experimental drug has proved effective against the new coronavirus in a major U.S. government study that put it to a strict test.