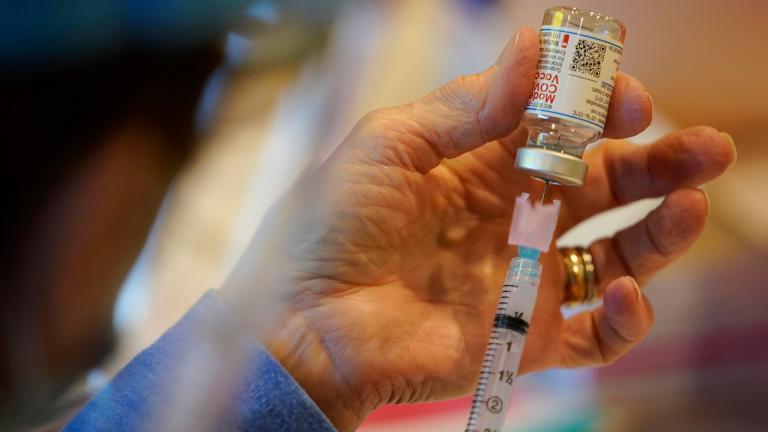
Moderna said Tuesday its COVID-19 vaccine strongly protects kids as young as 12, a step that could put the shot on track to become the second option for that age group in the U.S.
Moderna
Workers on Sunday began packaging shipments of the second COVID-19 vaccine authorized in the U.S., a desperately needed boost to efforts to bring the coronavirus pandemic under control.
Much-needed doses are set to arrive Monday after the Food and Drug Administration authorized an emergency rollout of the vaccine developed by Moderna Inc. and the National Institutes of Health.
The FDA’s green light for emergency use is expected quickly. Moderna would then begin shipping millions of doses, earmarked for health workers and nursing home residents, to boost the largest vaccination effort in U.S. history.
If the FDA allows emergency use, Moderna expects to have 20 million doses ready for the U.S. by year’s end. Recipients will need two doses, so that’s enough for 10 million people.
A second experimental COVID-19 vaccine — this one from Moderna Inc. — yielded extraordinarily strong early results Monday, another badly needed dose of hope as the pandemic enters a terrible new phase.