With many Americans who got Pfizer vaccinations already rolling up their sleeves for a booster shot, millions of others who received the Moderna or Johnson & Johnson vaccine wait anxiously to learn when it's their turn.
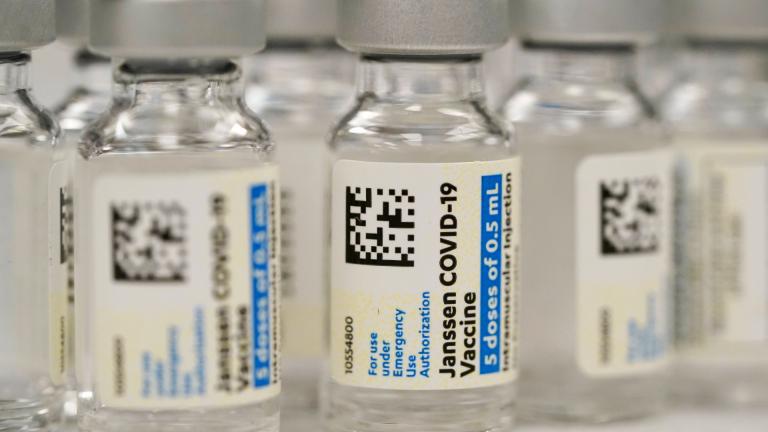
Johnson & Johnson
Johnson & Johnson said Tuesday that a booster of its one-shot coronavirus vaccine provides a stronger immune response months after people receive a first dose.
U.S. regulators on Monday added a new warning to Johnson & Johnson’s COVID-19 vaccine about links to a rare and potentially dangerous neurological reaction, but said it’s not entirely clear the shot caused the problem.
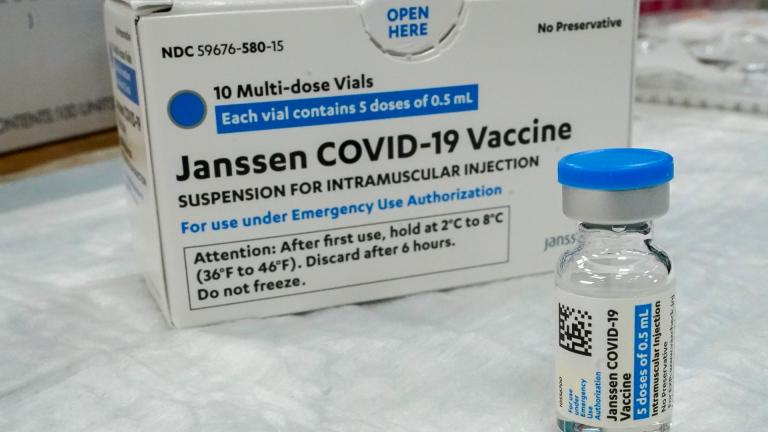
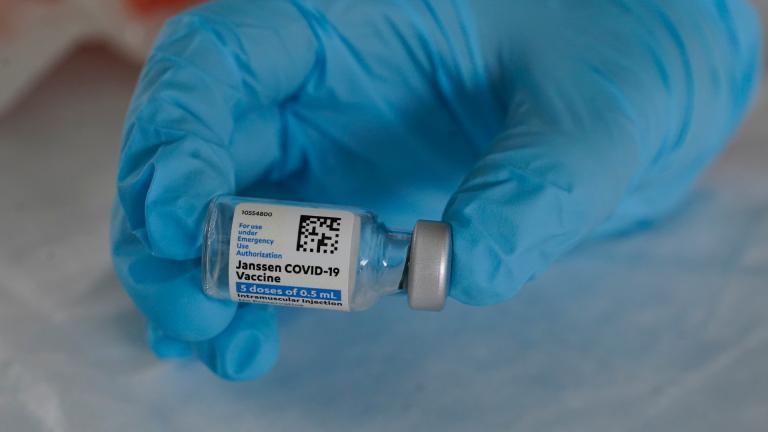
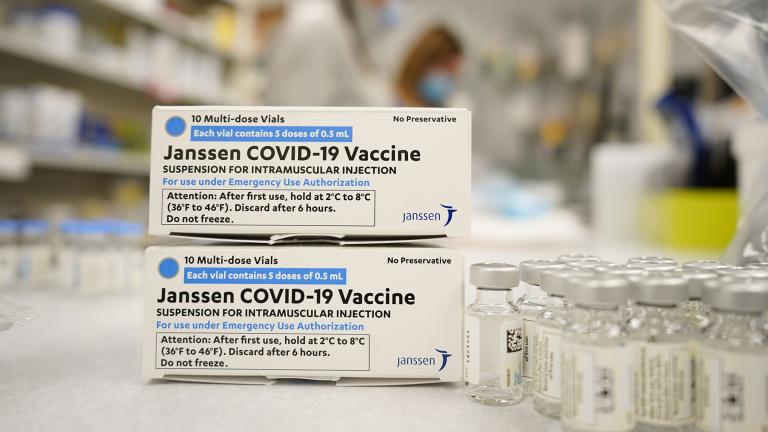
The company said a Food and Drug Administration review concluded the shots remain safe and effective for at least 4 1/2 months. In February, the FDA originally authorized J&J’s vaccine for up to three months when stored at normal refrigeration levels.
The Supreme Court is leaving in place a $2 billion verdict in favor of women who claim they developed ovarian cancer from using Johnson & Johnson talc products.
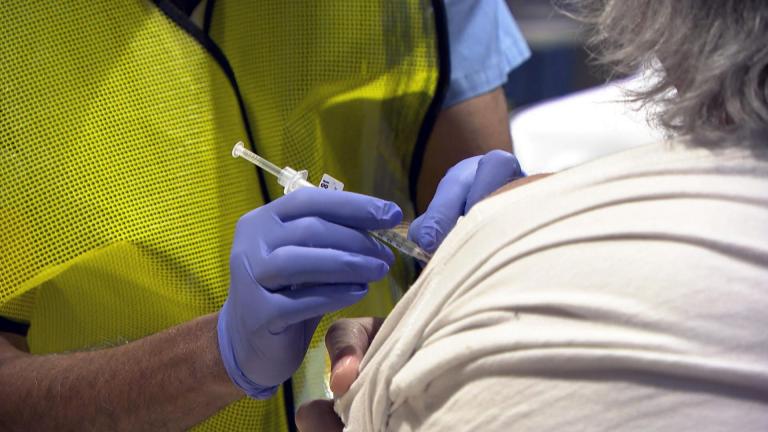
With a green light from federal health officials, many states resumed use of the one-shot Johnson & Johnson coronavirus vaccine on Saturday. Among the venues where it was being deployed: the Indianapolis Motor Speedway.
The Illinois Department of Public Health, the Chicago Department of Public Health and the Cook County Department of Public Health restarted administering the Johnson & Johnson vaccine on Saturday, as officials said it is safe and effective.
U.S. health officials lifted an 11-day pause on COVID-19 vaccinations using Johnson & Johnson's single-dose shot on Friday, after scientific advisers decided its benefits outweigh a rare risk of blood clot.
The United States will likely move to resume Johnson & Johnson’s COVID-19 vaccine this coming week, possibly with restrictions or broader warnings after reports of some very rare blood clot cases, the government’s top infectious diseases expert said Sunday.
Health officials recommended a pause on the Johnson & Johnson COVID-19 vaccine this week after six people experienced rare but severe blood clots. We discuss the situation—and concerns about vaccine hesitancy—with Dr. Juanita Mora, an allergist and immunologist at the Chicago Allergy Center.
Johnson & Johnson’s COVID-19 vaccine will remain in limbo for a while longer after government health advisers declared Wednesday that they need more evidence to decide if a handful of unusual blood clots were linked to the shot — and if so, how big the risk really is.
The U.S. on Tuesday recommended a “pause” in use of the single-dose Johnson & Johnson COVID-19 vaccine to investigate reports of rare but potentially dangerous blood clots, setting off a chain reaction worldwide and dealing a setback to the global vaccination campaign.
Health officials said they were acting “out of an abundance of caution” following six cases of a rare and severe type of blood clot in individuals who got the one-dose Johnson & Johnson vaccine.
The announcement comes as the White House looks to speed the production of the single-dose vaccine. Officials have said J&J faced unexpected production issues with its vaccine and produced only 3.9 million doses ahead of its receiving emergency use authorization on Saturday.
The U.S. is getting a third vaccine to prevent COVID-19, as the Food and Drug Administration on Saturday cleared a Johnson & Johnson shot that works with just one dose instead of two.
U.S. health advisers endorsed a one-dose COVID-19 vaccine from Johnson & Johnson on Friday, putting the nation on the cusp of adding an easier-to-use option to fight the pandemic.